Colibrí™, COPAN’s Automated Specimen Workup Instrument, Receives FDA 510(k) Clearance for use with bioMérieux VITEK® 2 Antimicrobial Susceptibility Testing (AST) System
October 10, 2022


Copan is pleased to announce we've received 510(k) clearance K232357 for our Universal Transport Media (UTM) without beads, specifically for molecular testing applications. As part of this update, we're optimizing our product portfolio by consolidating certain SKUs to improve efficiency and availability. This FAQ provides details about compliant product codes, alternatives for discontinued items, transition timelines, and answers to common questions about the impact on laboratory workflows.
March 13, 2022
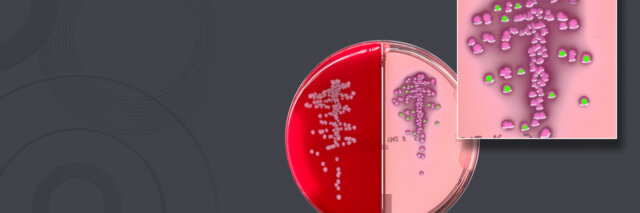
COPAN Diagnostics Announces Another Giant Step in the Evolution of Artificial Intelligence in Clinical Microbiology
January 19, 2022
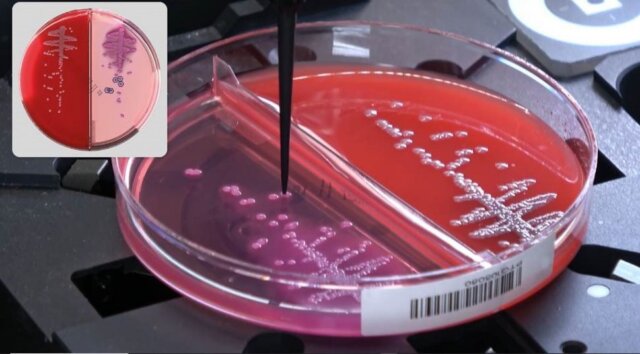
Colibri™ Automated Specimen Workup Instrument by COPAN Receives FDA 510(k) Clearance for Use with MALDI-TOF Mass Spectrometry Analyzers
January 5, 2022