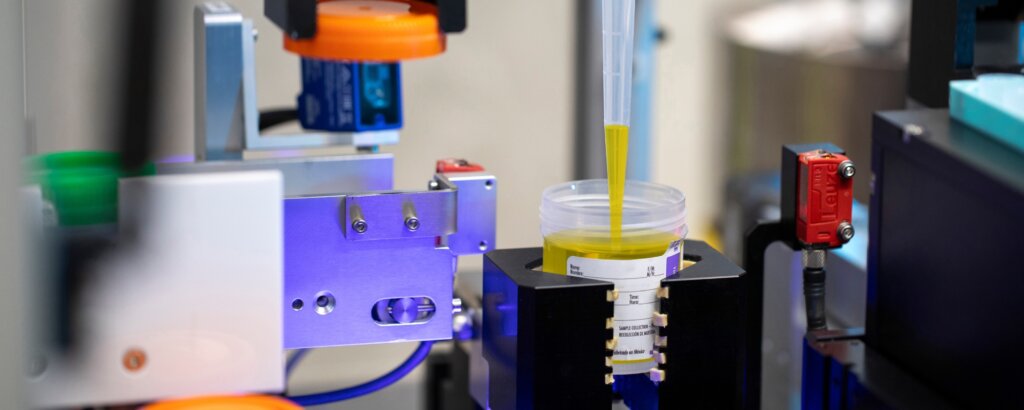
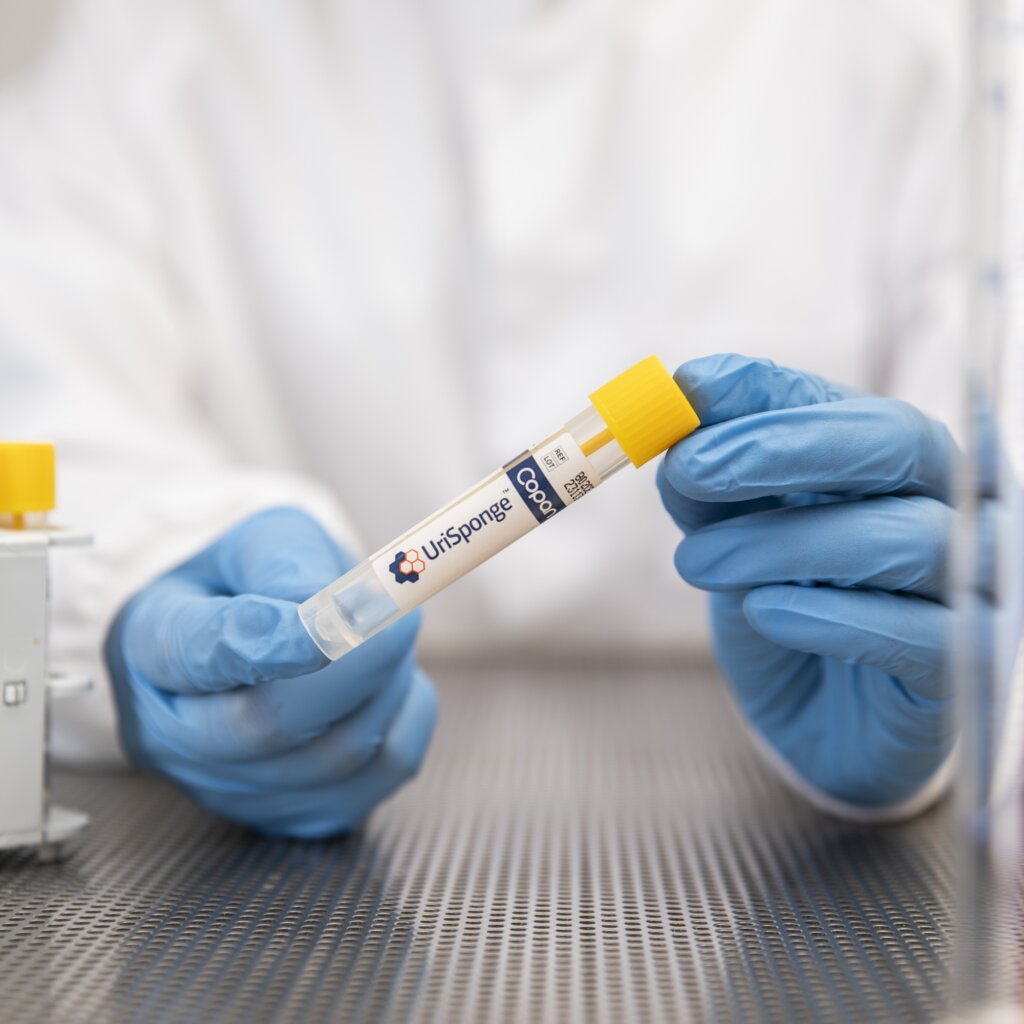
FDA Clears UriSponge®: A Streamlined, Cost-Effective Solution for Urine Specimen Collection and Transport
 Read More
Read More


Copan Colibrí™ Receives Third FDA Clearance, Further Expanding Automated ID/AST Workup Capabilities
January 30, 2024



Colibrí™, COPAN’s Automated Specimen Workup Instrument, Receives FDA 510(k) Clearance for use with bioMérieux VITEK® 2 Antimicrobial Susceptibility Testing (AST) System
October 10, 2022