FDA Clears UriSponge®: A Streamlined, Cost-Effective Solution for Urine Specimen Collection and Transport
December 9, 2024
Key Insights:
- FDA Clearance: The newly formulated UriSponge® is now FDA-cleared, affirming its reliability and effectiveness for urine specimen collection and preservation.
- Time and Workflow Efficiency: The dip-and-close design simplifies collection, reducing handling time and minimizing errors.
- Cost Effective: Reduces dependency on consumables like transfer straws and suction needles, streamlining processes and lowering material costs.
- Automation-Ready: Seamlessly integrates with full laboratory automation such as the Copan WASP® system, supporting high-throughput workflows in clinical laboratories.
Estimated Reading Time: 8 Minutes
Introduction
Urine specimen collection and preservation are critical aspects of accurate clinical diagnostics. UriSponge® offers a streamlined, cost-effective, and FDA-cleared solution for improved urine specimen management.
Copan Diagnostics is pleased to announce that its innovative urine collection and transport device, UriSponge®, has received clearance from the U.S. Food and Drug Administration (FDA). This significant milestone underscores the reliability and effectiveness of UriSponge® in enhancing urine specimen collection and preservation processes.
FDA Clearance Brings UriSponge® to Clinical Laboratories in the United States
The FDA clearance of UriSponge® highlights its potential to significantly improve workflow efficiency in laboratories. The system’s dip-and-close design allows for quick and simple urine specimen collection, reducing handling time and lowering the risk of errors.
UriSponge® also reduces the need for consumables such as suction needles and transfer devices, leading to cost savings by minimizing waste and lowering material costs. This efficiency makes it an ideal solution for laboratories aiming to maximize throughput while maintaining a high standard of specimen integrity.
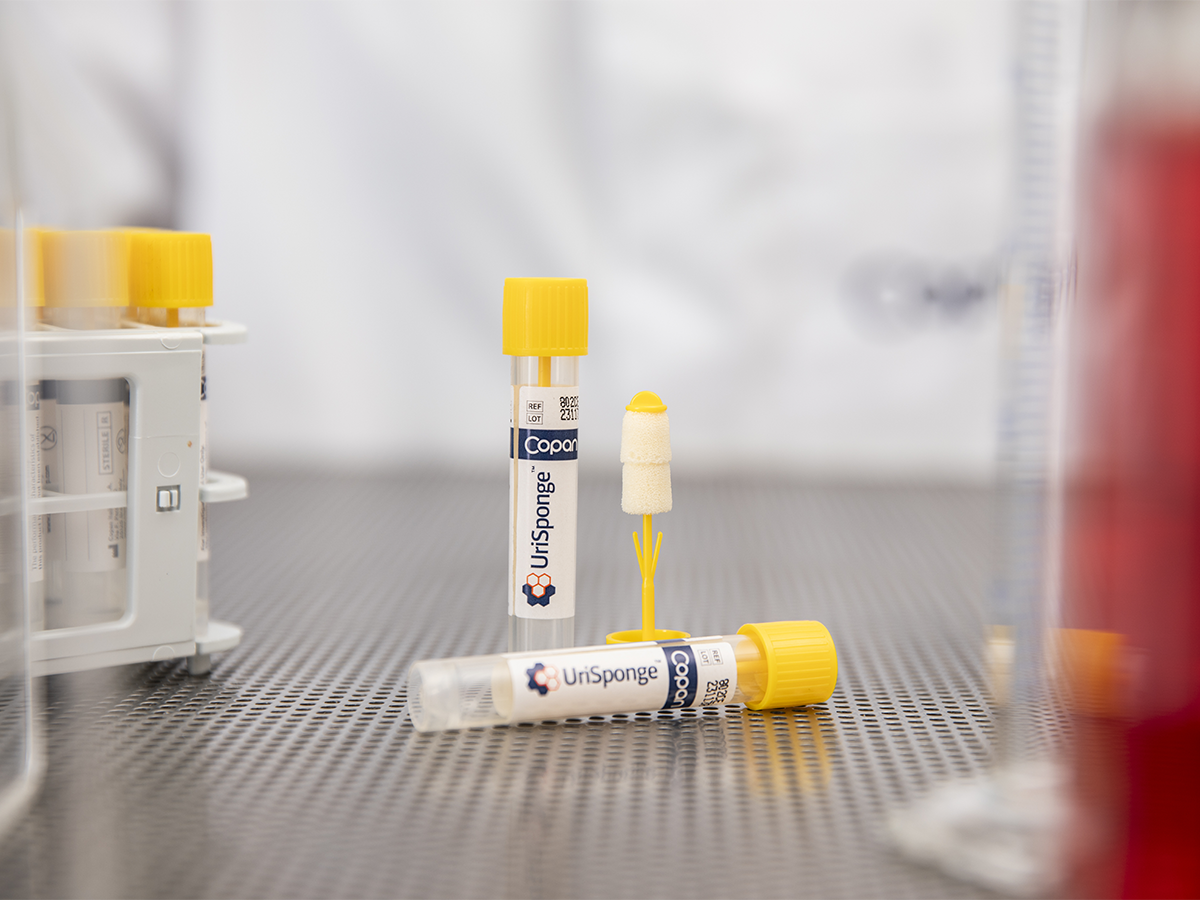
Reliable Specimen Preservation Without Fill Lines
UriSponge® eliminates the need for a precise fill line, instead using a boric acid-free preservative system loaded into the absorbent sponges. This design ensures the correct urine-to-preservative ratio, maintaining specimen stability of indicated organisms for up to 48 hours under room or refrigerated conditions. By reliably preserving specimens without complex handling, UriSponge® helps ensure accurate diagnostics and minimizes the potential for specimen rejection.

Automation-Ready for High-Throughput Laboratories
UriSponge® is compatible with laboratory automation systems, including Copan’s WASP® Walk-Away Specimen Processor. Copan’s automated system supports hands-free planting and streaking, making it an ideal choice for high-throughput environments that rely on automated workflows.

Conclusion
The FDA clearance of UriSponge® marks an important milestone for Copan Diagnostics, validating the product’s design, safety, and efficacy for clinical use. UriSponge® meets the growing needs of laboratories by providing a streamlined, cost-effective, and automation-ready solution for urine specimen collection. With this clearance, Copan is set to support U.S. laboratories with a product that is cost-effective, simplifies workflows, and is automation-ready.
About Copan Diagnostics, Inc.
Copan Diagnostics, part of Copan Group, is a global leader in specimen collection, transport, and laboratory automation. Through its collaborative approach, Copan has developed breakthrough technologies such as FLOQSwabs®, ESwab®, UTM® Universal Transport Medium™, and Full Laboratory Automation and artificial intelligence. Copan continues to innovate and transform Collection and Transport systems, as well as laboratory automation, helping healthcare providers improve healthcare patient outcomes.