Study Validates Effectiveness of Copan FecalSwab® for Enteric Pathogen Detection on the BD MAX™ System
August 26, 2024
Key Insights:
- Study Validation: Researchers from McMaster University and St. Joseph’s Healthcare validate Copan’s FecalSwab® system with the BD MAX™ platform for detecting enteric pathogens.
- Performance and Sensitivity: FecalSwab® matched the sensitivity of traditional bulk stool samples with no significant differences in pathogen detection and showed enhanced detection in samples with low pathogen concentrations.
- Storage and Stability: Demonstrated effective preservation of viral and bacterial targets under various temperatures for up to two weeks, supporting its use in varied clinical environments and simplifying specimen transport logistics.
Estimated Reading Time: 5 Minutes
Introduction
At Copan, we are dedicated to developing innovative solutions that streamline and enhance microbiological sample collection, transport, and preservation. A recent study published in the Journal of Clinical Microbiology provides strong support for the use of Copan’s FecalSwab® system with the BD MAX™ platform for enteric pathogen detection.
Study Design
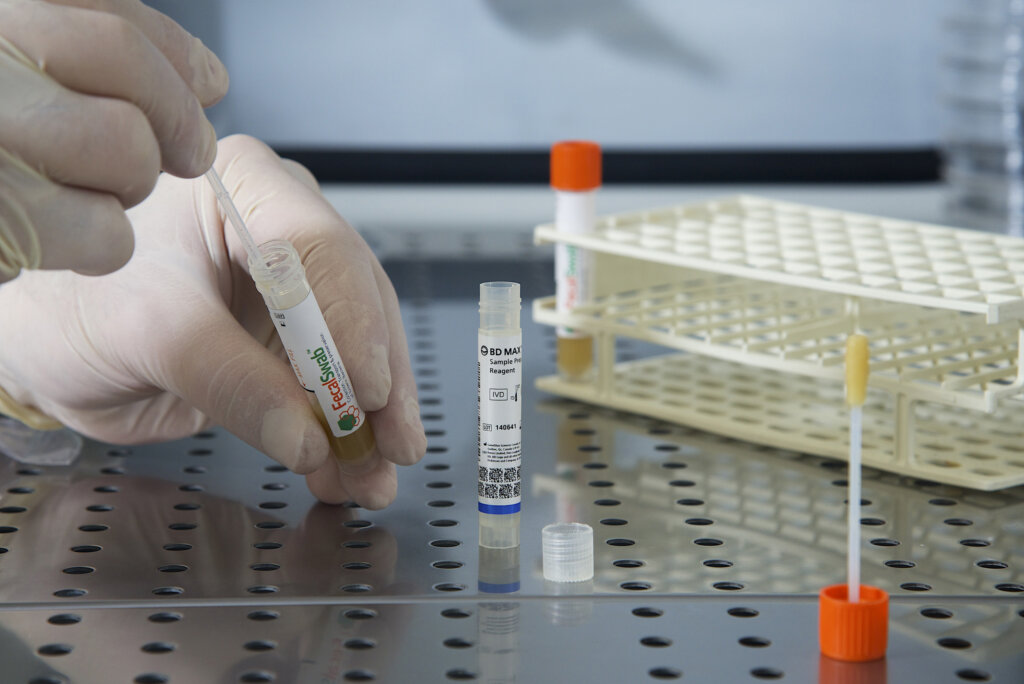
The study, led by researchers from McMaster University and St. Joseph’s Healthcare in Hamilton, Canada, evaluated the performance of FecalSwab® compared to bulk stool samples for detecting enteric viruses and bacteria on the BD MAX™ system. The researchers collected 186 unpreserved stool specimens and prepared matched FecalSwab® and bulk stool aliquots for testing.
Equivalent Performance to Bulk Stool
Using the BD MAX™ enteric viral panel (EVP) and bacterial panel (EBP) assays, the study found no significant differences in overall detection of viral and bacterial pathogens between 50 µL of FecalSwab® sample and the standard bulk stool inoculation volumes. Agreement between FecalSwab® and bulk stool was 99.3% for the EVP and 99.5% for the EBP, demonstrating that FecalSwab® provides equivalent sensitivity to the gold standard.
Improved Detection in Low Positive Samples
Interestingly, in the 7 samples with discordant results, the pathogen was detected in the FecalSwab® sample but not the bulk stool. Further analysis revealed these samples had high cycle threshold (Ct) values, indicating low pathogen concentrations. This suggests that FecalSwab® may actually enhance detection in samples with lower target levels.
Enabling Convenient, Ambient Temperature Storage
To evaluate FecalSwab®’s capacity to preserve targets during transport, the researchers stored samples at 4°C, 22°C, and 35°C for up to 14 days. Viral targets remained stable in FecalSwab® at all temperatures over the two-week period, with the exception of astrovirus at the highest temperature at later timepoints.
While bacterial targets showed some variability at ambient temperatures, refrigerated FecalSwab® samples were stable for the full 14 days. These findings highlight the flexibility that FecalSwab® provides for specimen handling.
Optimized for Automation and Molecular Assays
With its liquid-based format, FecalSwab® is well-suited for automated specimen processing, minimizing hands-on time and manual steps. The study also reinforces FecalSwab®’s compatibility with multi-target molecular panels like those on the BD MAX™, supporting its use across a range of enteric testing applications.
Conclusion
This study by Richard-Greenblatt, et al, provides robust evidence that Copan’s FecalSwab® is a reliable and high-performing collection and transport system for enteric pathogens. By demonstrating equivalency to bulk stool, enhanced sensitivity for low positives, and stability at varied temperatures, the study showcases FecalSwab®’s versatility for both traditional culture and cutting-edge molecular methods. At Copan, we’re proud to offer solutions like FecalSwab® that can help laboratories streamline workflow and optimize enteric disease diagnosis.
Research RoundUp: FecalSwab® and Molecular Testing
COPAN features a comprehensive review and commentary on recent scientific publications covering a particular topic of interest.
In this edition of Research Roundup, Dr. Susan Sharp summarizes research that explores FecalSwab® for manual and automated culture setup and enteric molecular diagnostics.

References:
- Richard-Greenblatt M, Rutherford C, Luinstra K, et al. Evaluation of the FecalSwab for Stool Specimen Storage and Molecular Detection of Enteropathogens on the BD Max System. J Clin Microbiol. 2020 Sep 24;58(9):e00178-20. doi: 10.1128/JCM.00178-20
About Copan Diagnostics
Copan is the leading innovator in sample collection, transport, and preservation systems. For over 40 years we have collaborated with laboratories to create breakthrough technologies advancing traditional and contemporary assays.
Explore our complete range of products powering clinical, industrial, forensics, genetics, and research workflows.