FecalSwab® FAQ
This FAQ page provides information on FecalSwab®, a collection, transport and processing system for enteric bacteria. It covers topics such as what FecalSwab® is, how to use it, applications, and availability.
Contents
- What is FecalSwab®?
- What is FecalSwab® used for?
- How is FecalSwab® Used?
- What are the different formats of FecalSwab® for fecal sample collection?
- How does FecalSwab® simplify fecal sampling?
- How long are samples stable in the FecalSwab® transport system?
- How does FecalSwab® ensure the viability of pathogens?
- What is Cary-Blair media?
- What are the benefits of using a flocked swab as part of the FecalSwab® sample collection and transport system?
- What pathogens can be detected from samples collected in FecalSwab®?
- Can FecalSwab® be used for pediatric samples?
- Can FecalSwab® be used as a stool sample kit for C. difficile testing?
- Is FecalSwab® compatible with Cary-Blair based enteric pathogen panels?
- Has FecalSwab® been cleared by the FDA for use with molecular enteric testing platforms?
- How does FecalSwab® compare to traditional stool collection methods for molecular testing?
- Is FecalSwab® suitable for infection control surveillance of multidrug-resistant organisms (MDROs)?
- Why is it Important to Mash the Specimen with the Swab?
- Can FecalSwab® Be Used With Automation?
- How is FecalSwab® disposed of after use?
- What research supports the use of FecalSwab®?
- Where Can I Order FecalSwab®?
- Disclaimers & References
- Do You Have Other Questions about FecalSwab® FAQ?
What is FecalSwab®?
FecalSwab® is a patented sample collection and preservation system designed to simplify the collection, transport, and preservation of stool samples for the detection of enteric bacteria and pathogens. It pairs FLOQSwabs® (Copan-invented flocked swabs consisting of Nylon fibers) with liquid Cary-Blair medium in a compact, easy-to-use format. Converting semi-solid fecal matter into liquid specimens simplifies and standardizes fecal sample collection, transport, and processing.

What is FecalSwab® used for?
FecalSwab® is an innovative fecal transport system designed for the convenient collection and transport of fecal samples for enteric pathogen testing. It maintains the viability of enteric bacteria during transport from the collection site to the laboratory.

How is FecalSwab® Used?
Using the Flocked Swab provided in the collection kit, a small sample of feces (approx 1g) can be transferred into the tube by medical staff at the point of care or by a technologist after the fecal specimen has been received in the laboratory. Alternatively, FecalSwab® can be used to take a rectal sample directly from the patient.
Using the Flocked Swab provided in the collection kit, a small sample of feces (approx 1g) can be transferred into the tube by medical staff at the point of care or by a technologist after the fecal specimen has been received in the laboratory. Alternatively, FecalSwab® can be used to take a rectal sample directly from the patient.

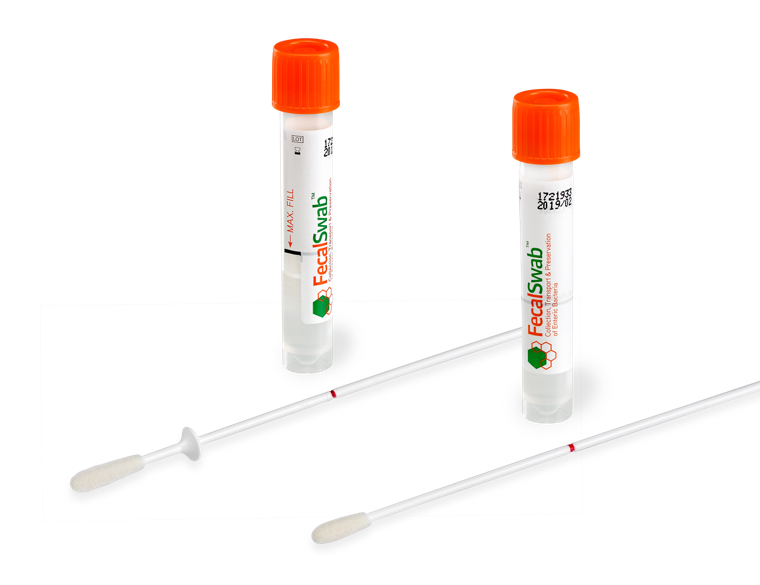
What are the different formats of FecalSwab® for fecal sample collection?
FecalSwab® offers flexibility in fecal sampling with two swab configurations:
- Standard FLOQSwab® flocked swab for collection from bulk stool specimens
- FLOQSwab® flocked swab with stopper for standardized rectal sampling
Both formats utilize the same 2mL of Cary-Blair medium and tube for optimal preservation and transport to the lab.
How does FecalSwab® simplify fecal sampling?
FecalSwab® streamlines fecal sampling from collection to analysis. FecalSwab®’s compact design takes up less space and eliminates the need for bulky containers. The liquid Cary-Blair media enables compatibility with automated processors and pipettes. The flocked swabs provide efficient sample collection for both stool and rectal sampling.

How long are samples stable in the FecalSwab® transport system?
FecalSwab® preserves fecal specimens for up to 48 hours at room temperature (20-25°C) or up to 72 hours refrigerated (2-8°C). For C. difficile, samples are stable for 24 hours at room temperature or 48 hours refrigerated. Prompt transport to the laboratory is always recommended.
How does FecalSwab® ensure the viability of pathogens?
The Cary-Blair medium included in the FecalSwab® system helps maintain the viability of enteric pathogens by stabilizing them at a pH that prevents overgrowth and die-off. Independent studies have confirmed that the medium effectively preserves various pathogens under recommended storage conditions [1],[2],[3].
What is Cary-Blair media?
Cary-Blair media is a non-nutritive transport and preservation medium used for the collection and transport of clinical specimens. It is designed to maintain the viability of enteric pathogens during transport from the collection site to the testing laboratory. The medium is commonly used in conjunction with fecal sample collection devices, such as the FecalSwab®.
The medium contains a balanced mixture of salts, minerals, and a low nutrient content to prevent the overgrowth of commensal organisms while preserving the target pathogens. Cary-Blair media also includes a reducing agent and phosphate buffer to maintain a neutral pH, further promoting the survival of enteric bacteria.
What are the benefits of using a flocked swab as part of the FecalSwab® sample collection and transport system?
What pathogens can be detected from samples collected in FecalSwab®?
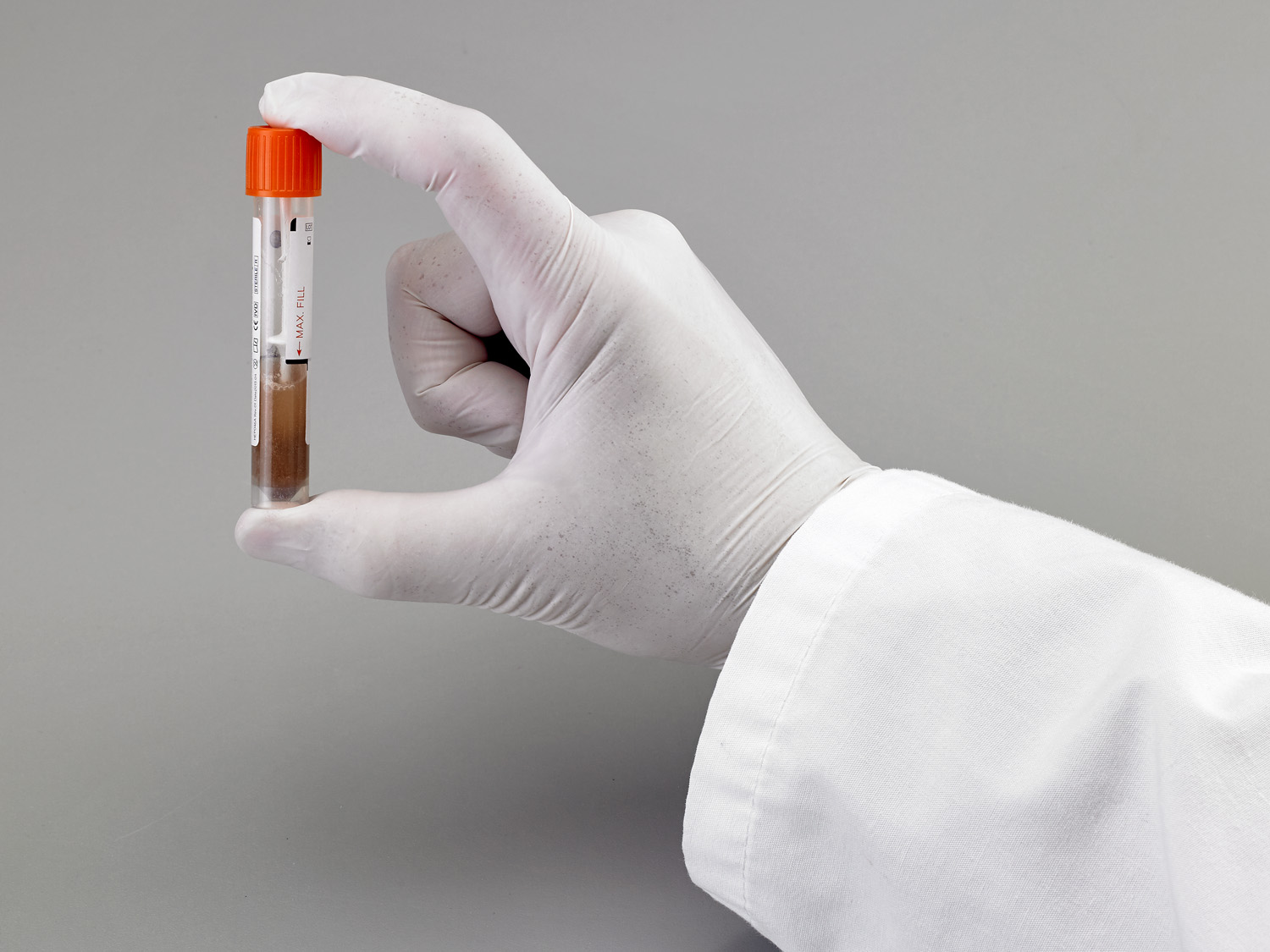
Can FecalSwab® be used for pediatric samples?
FecalSwab® provides a simplified and effective method for collecting pediatric stool samples. The system is specifically designed for the safe collection of rectal swabs, eliminating the need to rely on stool samples from diapers, which may be diluted by urine and compromise the quality of the fecal specimens [8].
Rectal swabs offer healthcare providers a convenient and reliable alternative for obtaining pediatric stool samples when a bulk stool culture is not possible. By utilizing a rectal swab, clinicians can collect samples promptly, potentially expediting the diagnostic process and enabling faster initiation of appropriate treatment [9],[10],[11].

Can FecalSwab® be used as a stool sample kit for C. difficile testing?
Yes, studies show FecalSwab® effectively preserves C. difficile for testing. Specimens should be refrigerated and processed within 48 hours for optimal C. difficile recovery. The liquid Cary-Blair medium maintains viability while preventing the overgrowth of other bacteria that can interfere with C. difficile detection**.
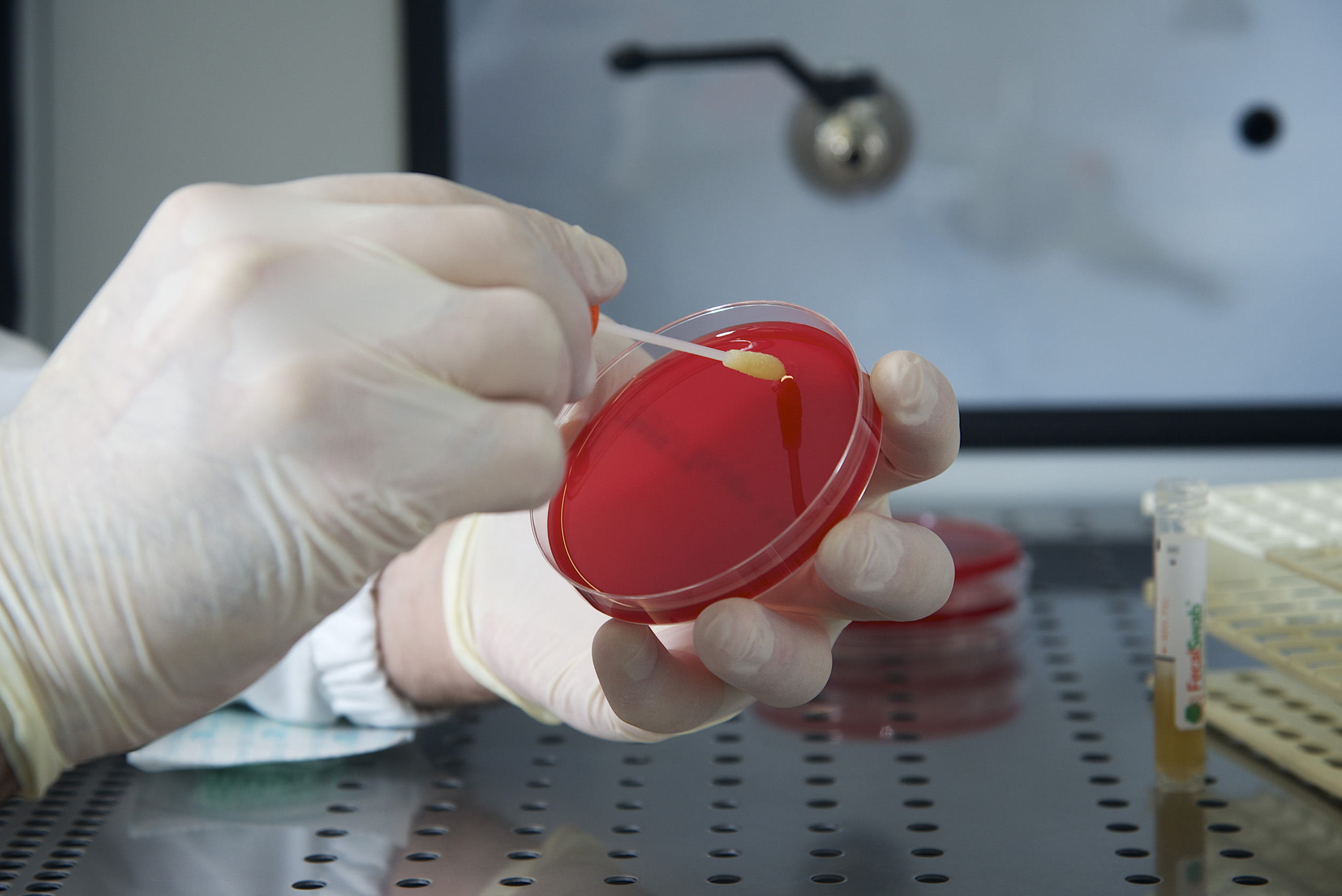
Is FecalSwab® compatible with Cary-Blair based enteric pathogen panels?
Yes, FecalSwab® contains a tube pre-filled with Cary-Blair transport medium. This non-nutritive medium contains balanced salts and amino acids to maintain microbe viability while inhibiting overgrowth. Its formulation is compatible with Cary-Blair based culture and molecular tests*.

Has FecalSwab® been cleared by the FDA for use with molecular enteric testing platforms?
In late 2022, FecalSwab® obtained FDA clearance for use with the BD MAX™ Enteric Bacterial Panel and BD MAX™ Extended Enteric Bacterial Panel. It is the only collection and transport device currently cleared by the FDA for molecular detection of enteropathogens using these BD MAX™ panels.
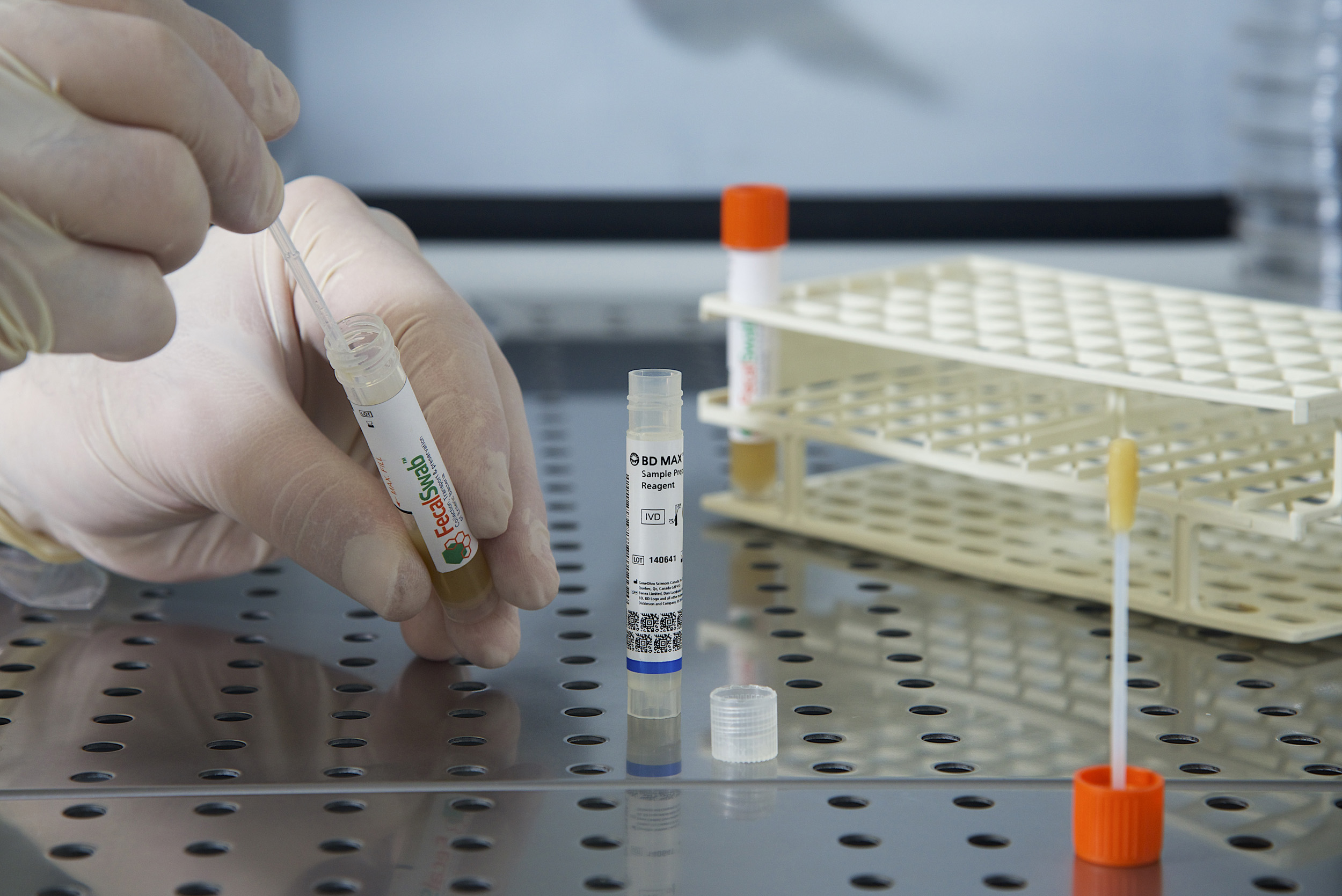
How does FecalSwab® compare to traditional stool collection methods for molecular testing?
Multiple studies have shown that FecalSwab® performs equivalently to bulk stool specimens or Cary-Blair media when used in various molecular assays, such as the Luminex Gastrointestinal Pathogen Panel [12], [13], BioFire FilmArray GI Panel [6], [10], BD MAX Enteric Bacterial Panel [4], and Cepheid Xpert C. difficile assay[14].

Is FecalSwab® suitable for infection control surveillance of multidrug-resistant organisms (MDROs)?
A study presented at ECCMID 2018 showed that FecalSwab® has a high sensitivity and specificity for rectal detection of extended-spectrum beta-lactamases (ESBLs), carbapenem-resistant Enterobacteriaceae (CRE), and C. difficile, making it a valuable tool for MDRO surveillance programs [15].

Why is it Important to Mash the Specimen with the Swab?
Mashing the specimen helps to evenly disperse and suspend the specimen ensuring a homogenous sample.
Can FecalSwab® Be Used With Automation?
Yes, FecalSwab® is packaged in plastic, ready-to-use tubes, which can easily be put onto WASP® automation specimen processor.

How is FecalSwab® disposed of after use?
Used FecalSwab® units should be considered biohazardous and disposed of according to your facility’s guidelines for biohazardous waste. Sterilize or properly contain all used materials to prevent infection and environmental contamination.
Like all clinical collection devices, FecalSwab® should be handled with standard biohazard precautions. The device is for single use to prevent contamination and infection risks. Always use aseptic technique when handling specimens.

What research supports the use of FecalSwab®?
Numerous studies have validated the efficacy of FecalSwab® for the preservation and transport of fecal specimens for enteric pathogen testing. These studies have demonstrated comparable detection rates to those obtained with fresh specimens, across a range of storage conditions.

Where Can I Order FecalSwab®?
FecalSwab® is Supplied by companies listed in our FecalSwab® Distributors Page below.

Disclaimers & References
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
** For recovery of C. difficile, FecalSwab specimens should be refrigerated at 2–8ºC and processed within 48 hours or stored at room temperature (20–25°C) and processed within 24 hours.


