UriSponge® FAQ
Explore the UriSponge® FAQ page to learn about our advanced, boric acid-free urine specimen preservation and transport system. Discover its uses, safety features, operational benefits, and how it streamlines workflows for modern laboratories.
Contents
- What is UriSponge® and how does it differ from traditional urine collection devices?
- How is UriSponge® different from traditional urine collection devices?
- What are the key benefits of UriSponge® for clinical laboratories?
- How is UriSponge® used?
- What are the operational benefits of UriSponge® over traditional collection methods?
- Is UriSponge® safe for healthcare workers?
- How does UriSponge® help reduce costs for clinical laboratories?
- What preservative does UriSponge® use for urine specimens?
- What are the main advantages of using a boric acid-free urine specimen transport system?
- How long does UriSponge® preserve urine samples, and how does it ensure integrity during transport?
- Can UriSponge® be stored at room temperature?
- What is the typical urine volume absorbed by UriSponge®?
- How does UriSponge® reduce the risk of sample rejection?
- What makes UriSponge® logistically superior, and does it offer environmental benefits?
- Does UriSponge® offer environmental benefits?
- How is UriSponge® transported and stored?
- How does UriSponge® integrate with laboratory automation systems?
- Is UriSponge® compatible with manual and automated workflows?
- Is there training available for laboratories transitioning to UriSponge®?
- Where can I get UriSponge®?
- Do You Have Other Questions about UriSponge® FAQ?
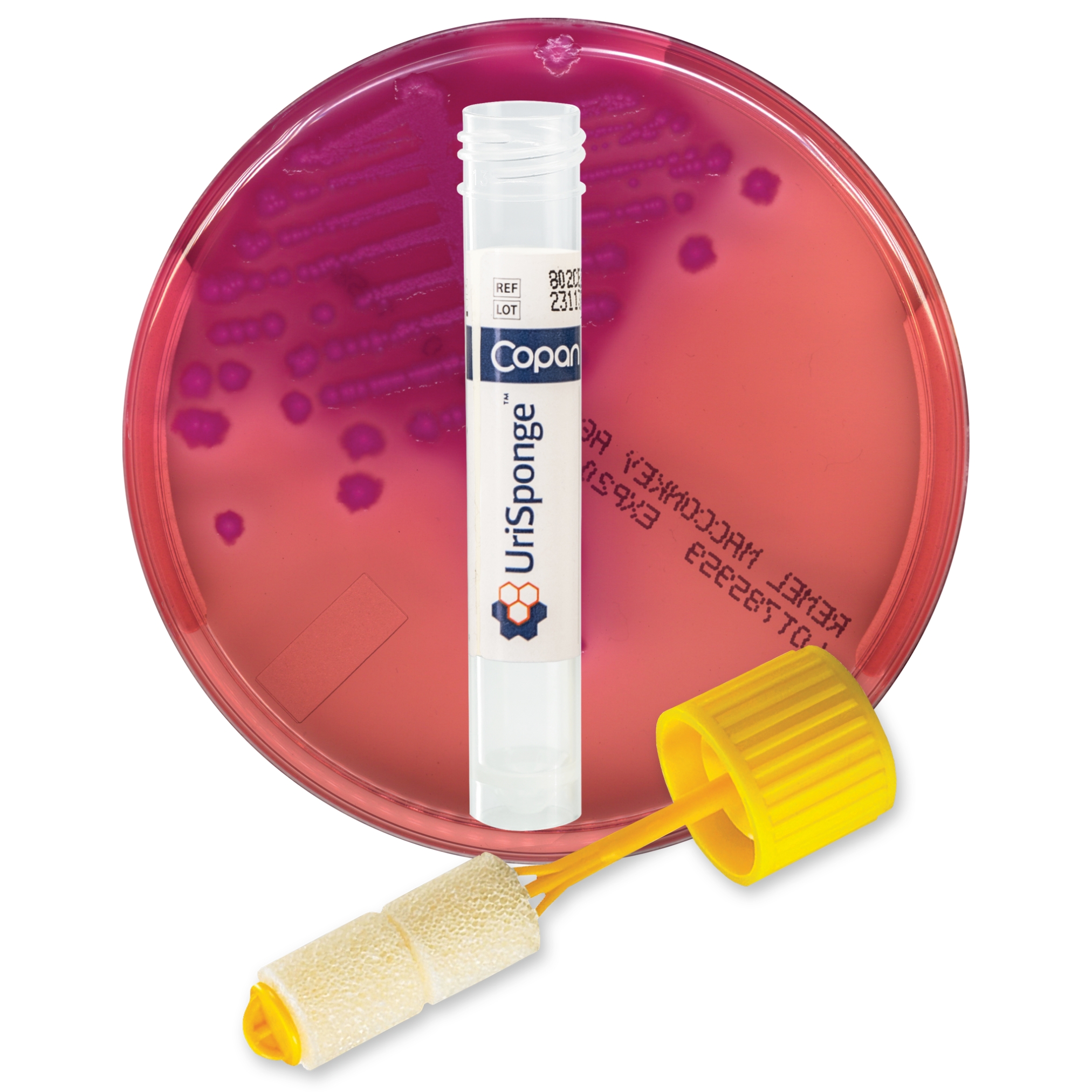
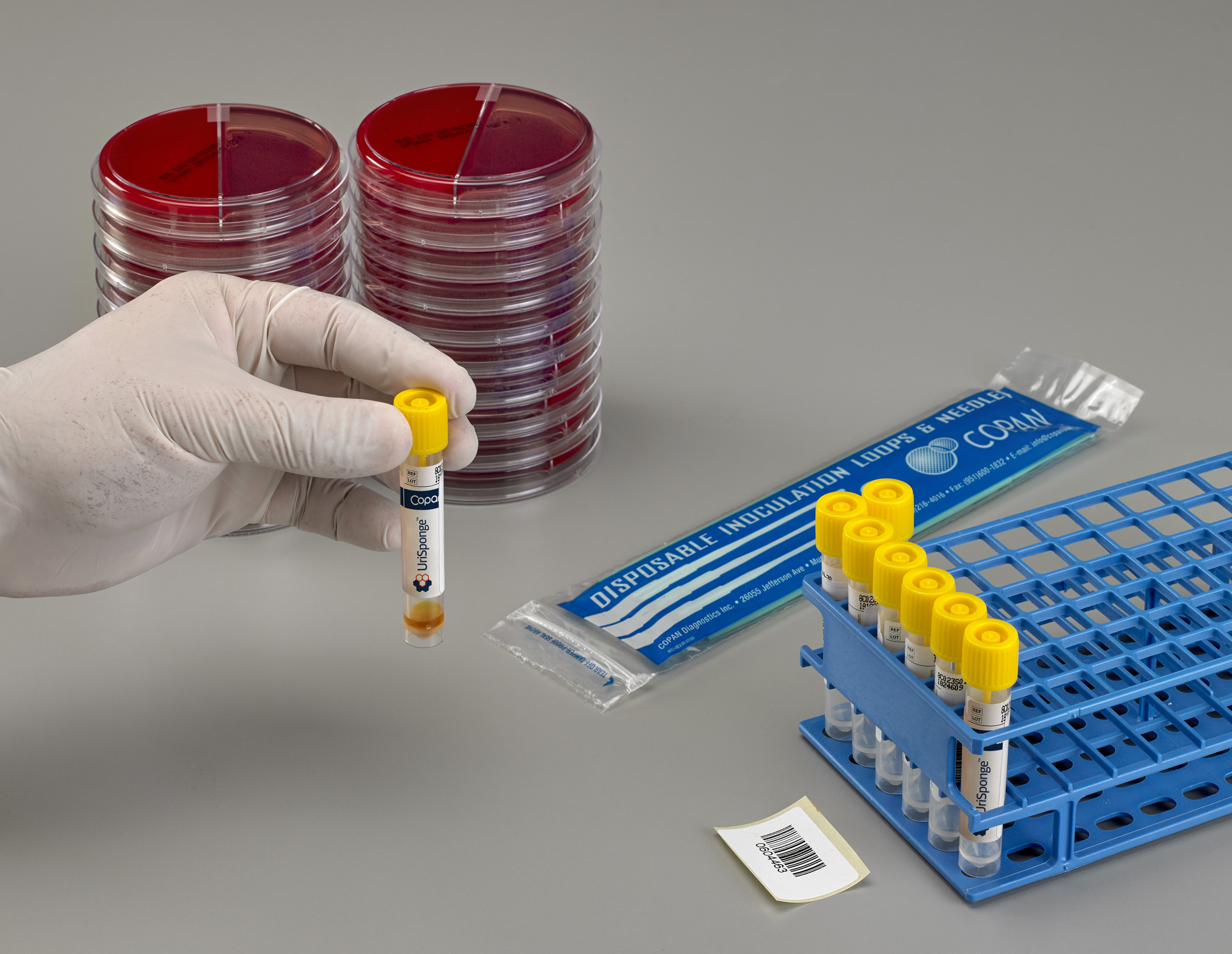
What is UriSponge® and how does it differ from traditional urine collection devices?
UriSponge® is an advanced urine specimen collection and transport device designed to simplify the collection process, preserve specimen stability, and enhance safety.
The system features a screw-cap tube with a plastic applicator stick and dual cylindrical sponges, simplifying urine collection. The device uses a unique, boric acid-free preservative blend to maintain specimen integrity for up to 48 hours and is compatible with automated laboratory workflows.
How is UriSponge® different from traditional urine collection devices?
UriSponge® is distinguished by its:
- Dip-and-close design, which simplifies the collection process.
- Needle-free mechanism, reducing the risk of sharps injuries.
- Boric acid-free preservative system (using sodium propionate and potassium sorbate)
- No fill line requirement, eliminating over- or under-filling concerns.
- Automation compatibility, allowing seamless integration with Copan’s WASP® Walk-Away Specimen Processor.
What are the key benefits of UriSponge® for clinical laboratories?
UriSponge® provides numerous advantages:
Workflow Efficiency: Simplifies collection with a dip-and-close design, reducing handling time and potential errors.
Cost Savings: Reduces dependency on consumables like suction needles, cutting down material costs and minimizing waste and waste disposal.
Automation Compatibility: Seamlessly integrates with Copan’s WASP® automation, enabling high-throughput laboratories to maintain productivity and reduce manual handling.
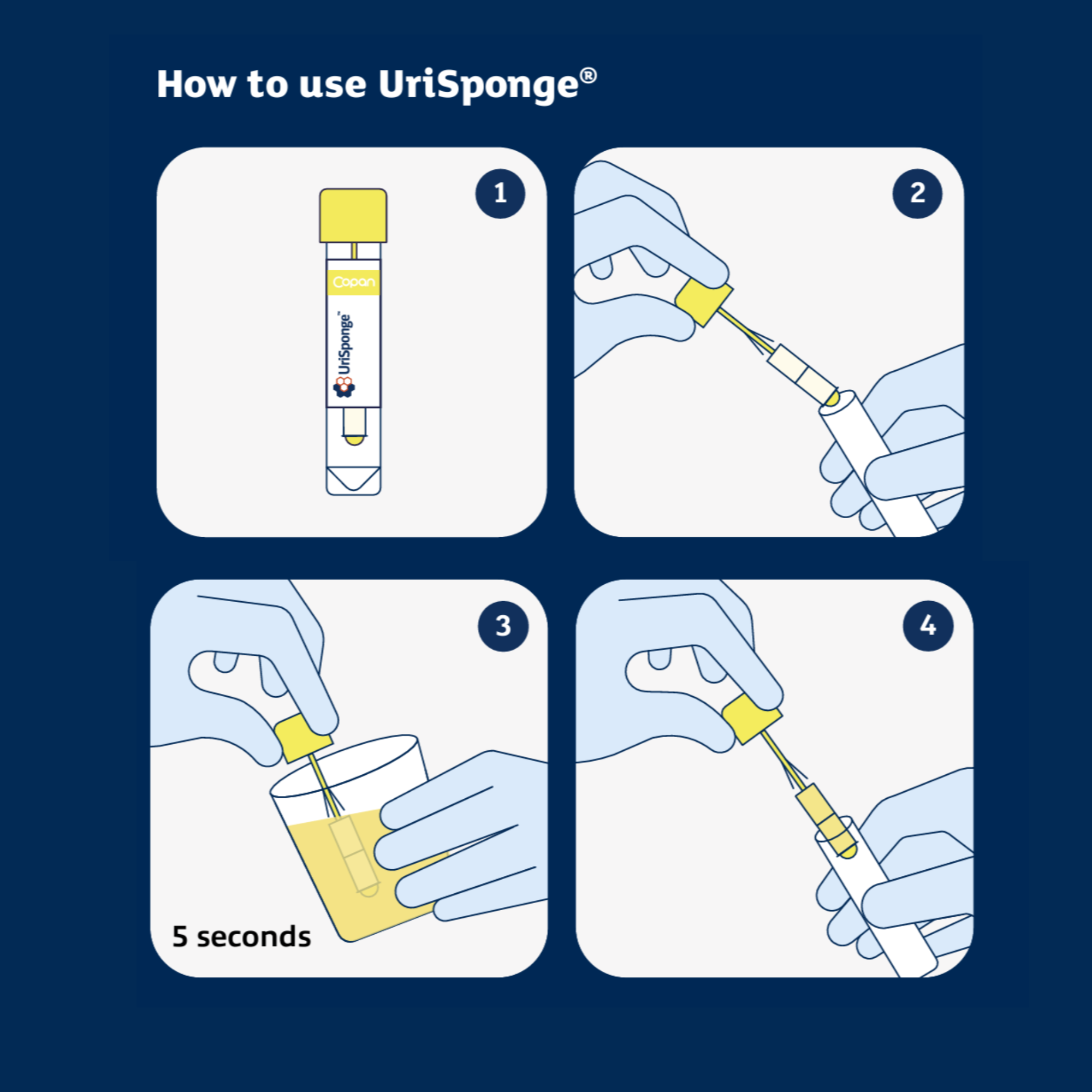
How is UriSponge® used?
Simply dip the sponge applicator into a clean-catch urine sample for 5 seconds. Return the sponge to the tube, securely close the cap, and send it to the laboratory. There’s no need for precise fill lines or additional consumables like suction needles or transfer straws.
What are the operational benefits of UriSponge® over traditional collection methods?
UriSponge® streamlines workflows by eliminating the need for suction needles and vacuum-sealed tubes, which require precise filling to preserve the urine specimen properly.
The quick sponge absorption process reduces collection time and minimizes the risk of errors, speeding up laboratory workflows and improving overall efficiency.
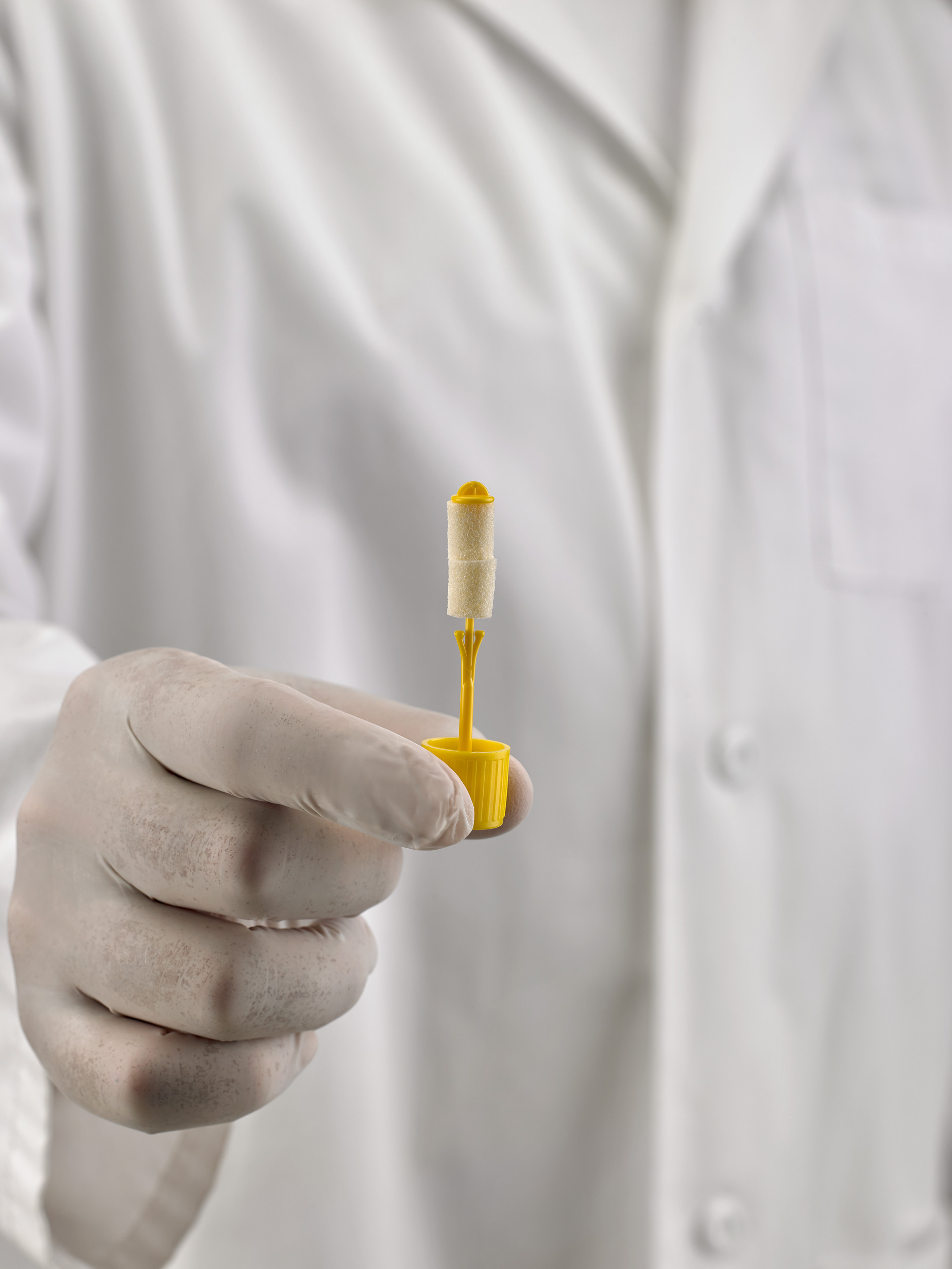
Is UriSponge® safe for healthcare workers?
Yes, UriSponge® is designed with safety in mind. By eliminating the need for suction needles.
UriSponge™ reduces the risk of sharps injuries, making it a safer and more efficient choice for healthcare workers.
How does UriSponge® help reduce costs for clinical laboratories?
UriSponge® reduces the need for additional consumables like suction needles and transfer devices, cutting down on material and waste disposal costs.
Its efficient preservative system, simple urine collection method, and lack of fill line also reduce the likelihood of specimen rejection, which can save time and resources.

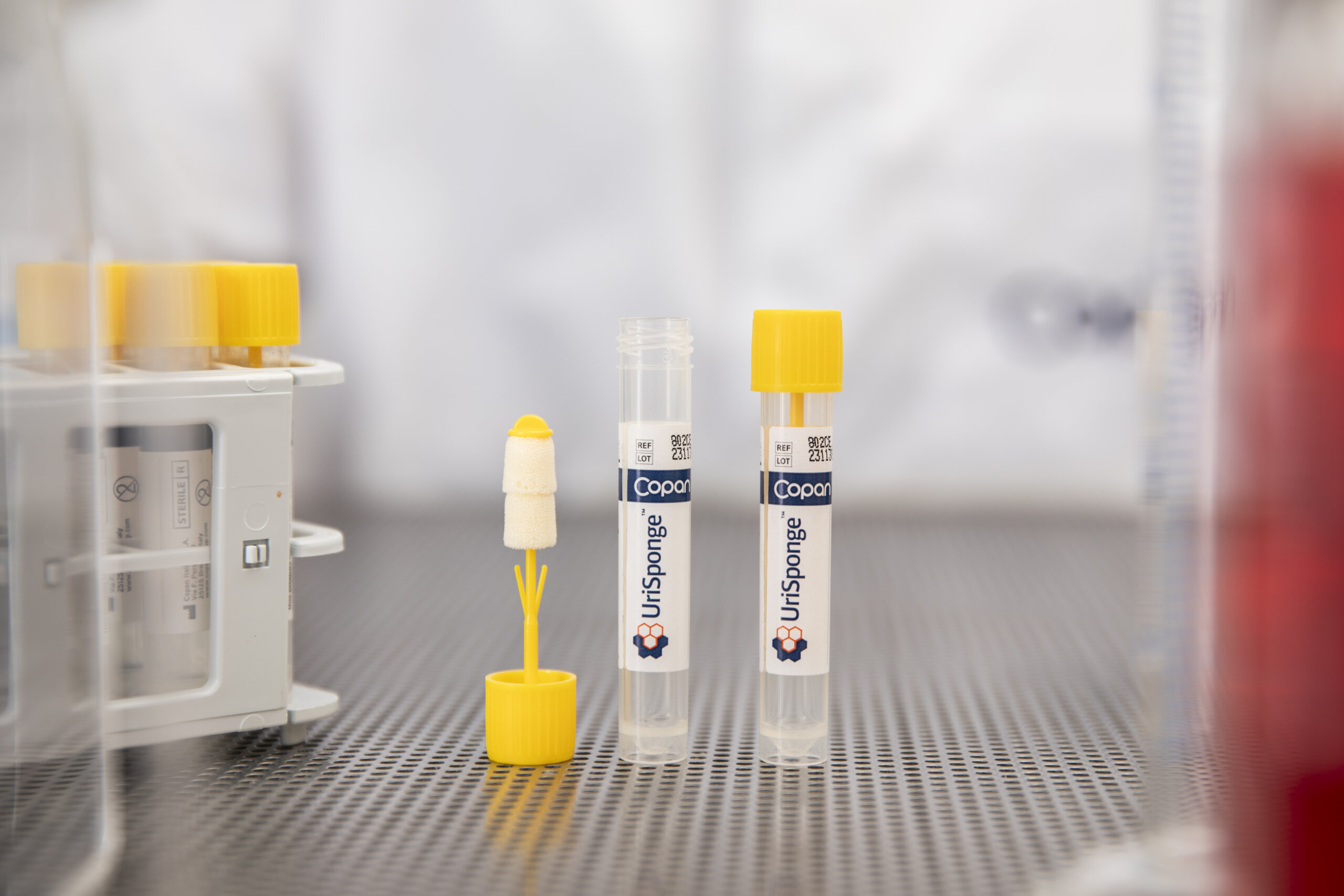
What preservative does UriSponge® use for urine specimens?
UriSponge® uses a boric acid-free preservative blend, consisting of sodium propionate and potassium sorbate, which ensures specimen stability for up to 48 hours at room or refrigerated temperatures.
This formulation supports safe and effective specimen preservation while meeting regulatory requirements for non-toxic alternatives to boric acid.

What are the main advantages of using a boric acid-free urine specimen transport system?
Boric acid-free systems, like UriSponge®, are safer in the manufacturing process and the environment, avoiding regulatory hurdles, chemical handling risks, and logistical delays associated with boric acid.
UriSponge® helps ensure sample preservation without the need for refrigeration, maintaining sample viability for up to 48 hours.
How long does UriSponge® preserve urine samples, and how does it ensure integrity during transport?
UriSponge® maintains the viability of bacterial specimens for up to 48 hours at both room temperature (20-25°C) and refrigerated conditions (4-8°C).
This extended stability period is ideal for laboratories with delayed processing times or long-distance transport needs. Its preservative system, upon contact with urine, maintains specimen stability throughout transport.

Can UriSponge® be stored at room temperature?
Yes, UriSponge® can be stored at room temperature (20-25°C) or refrigerated (2-8°C), maintaining sample viability for up to 48 hours, allowing flexibility in transport and storage.

What is the typical urine volume absorbed by UriSponge®?
UriSponge® sponges absorb approximately 1.0–1.5 mL of urine, which is sufficient for most laboratory tests without the need for precise fill lines.

How does UriSponge® reduce the risk of sample rejection?
UriSponge® minimizes the risk of collection errors by eliminating the need for precise fill lines and using absorbent sponges, leading to fewer rejected samples and fewer repeat collections.
What makes UriSponge® logistically superior, and does it offer environmental benefits?
UriSponge® simplifies logistics by eliminating hazardous boric acid, which has been indicated as a substance of high concern according to Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) Regulation.
By using an alternative preservative the UriSponge® may ease potential regulatory requirements, and reducing handling issues, resulting in fewer supply chain disruptions and faster delivery times.
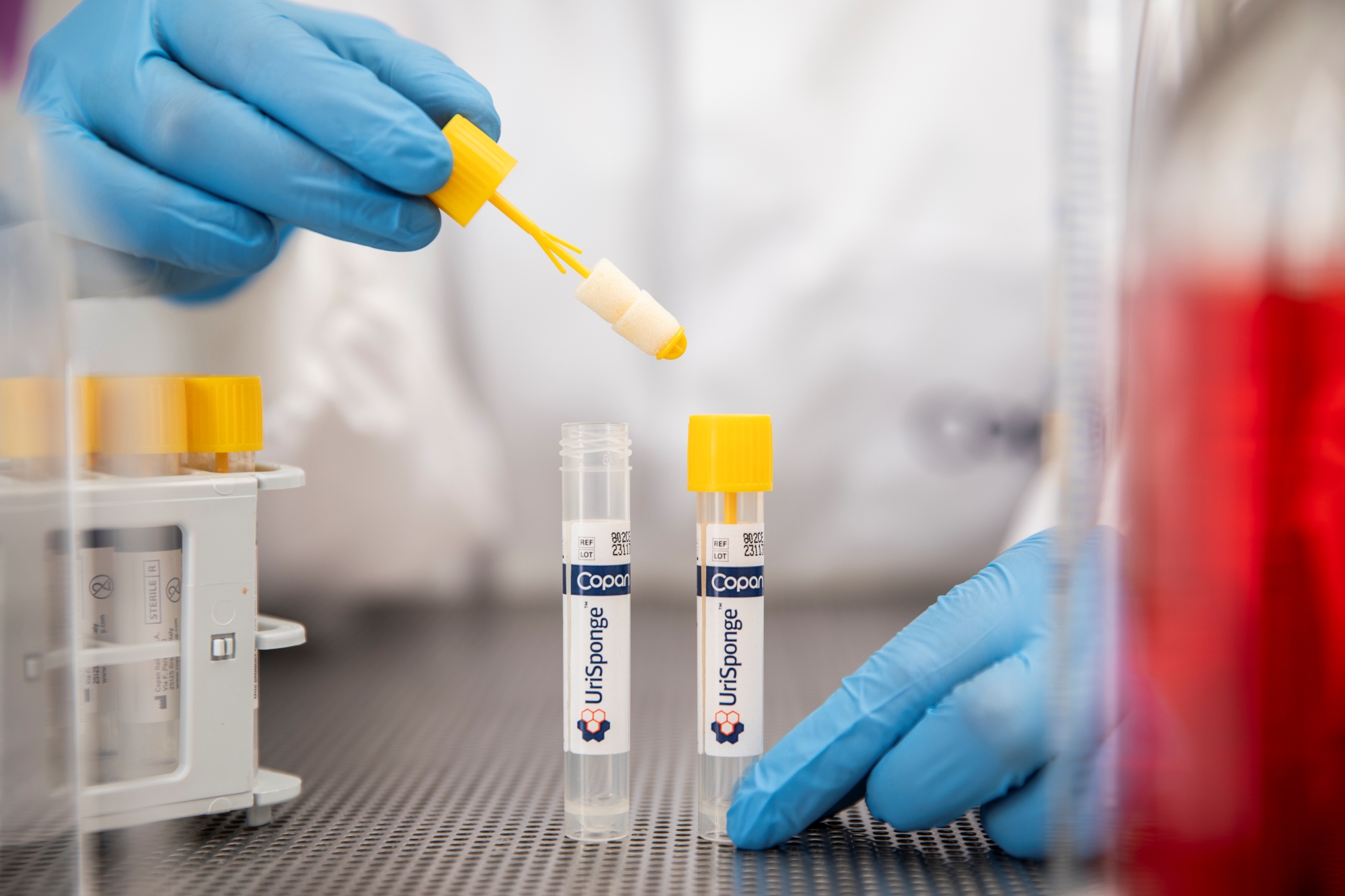
Does UriSponge® offer environmental benefits?
Environmentally, it reduces the use of hazardous chemicals, minimizes waste, and eliminates the need for additional materials like needles and straws. Additionally, its efficient design minimizes waste by removing the need for excess consumables like needles and transfer devices.
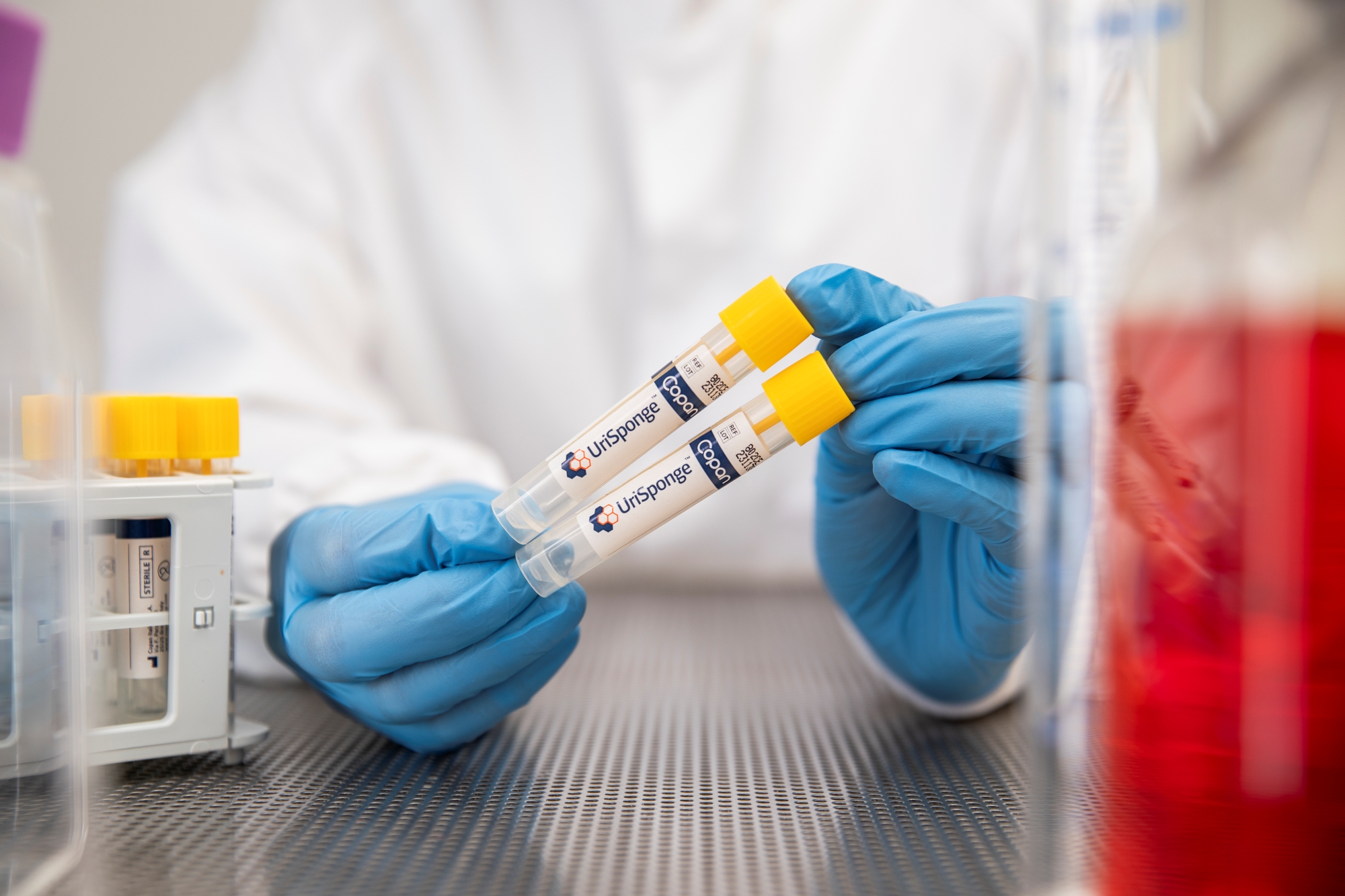
How is UriSponge® transported and stored?
UriSponge® can be transported and stored at room temperature or under refrigerated conditions, offering flexibility in logistics. Its preservative system ensures that specimen viability is maintained during extended storage or long-distance transport, making it suitable for diverse laboratory needs.
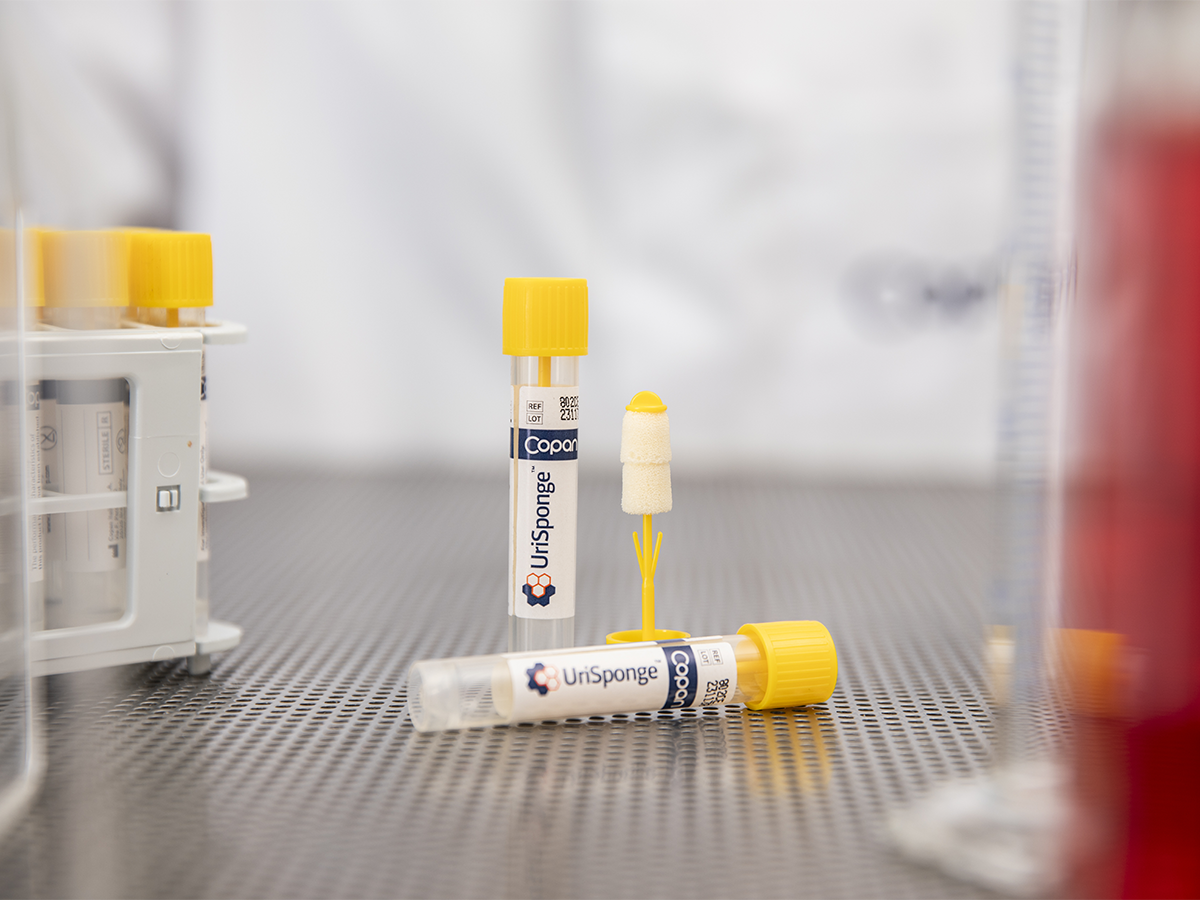
How does UriSponge® integrate with laboratory automation systems?
UriSponge® is fully compatible with laboratory automation such as Copan’s WASP® Walk-Away Specimen Processor, which automates specimen processing tasks such as planting and streaking.
This compatibility allows laboratories to incorporate UriSponge® into automated workflows, reducing manual handling and improving laboratory efficiency, particularly in high-volume environments.

Is UriSponge® compatible with manual and automated workflows?
Yes, UriSponge® is designed for both manual and automated workflows.
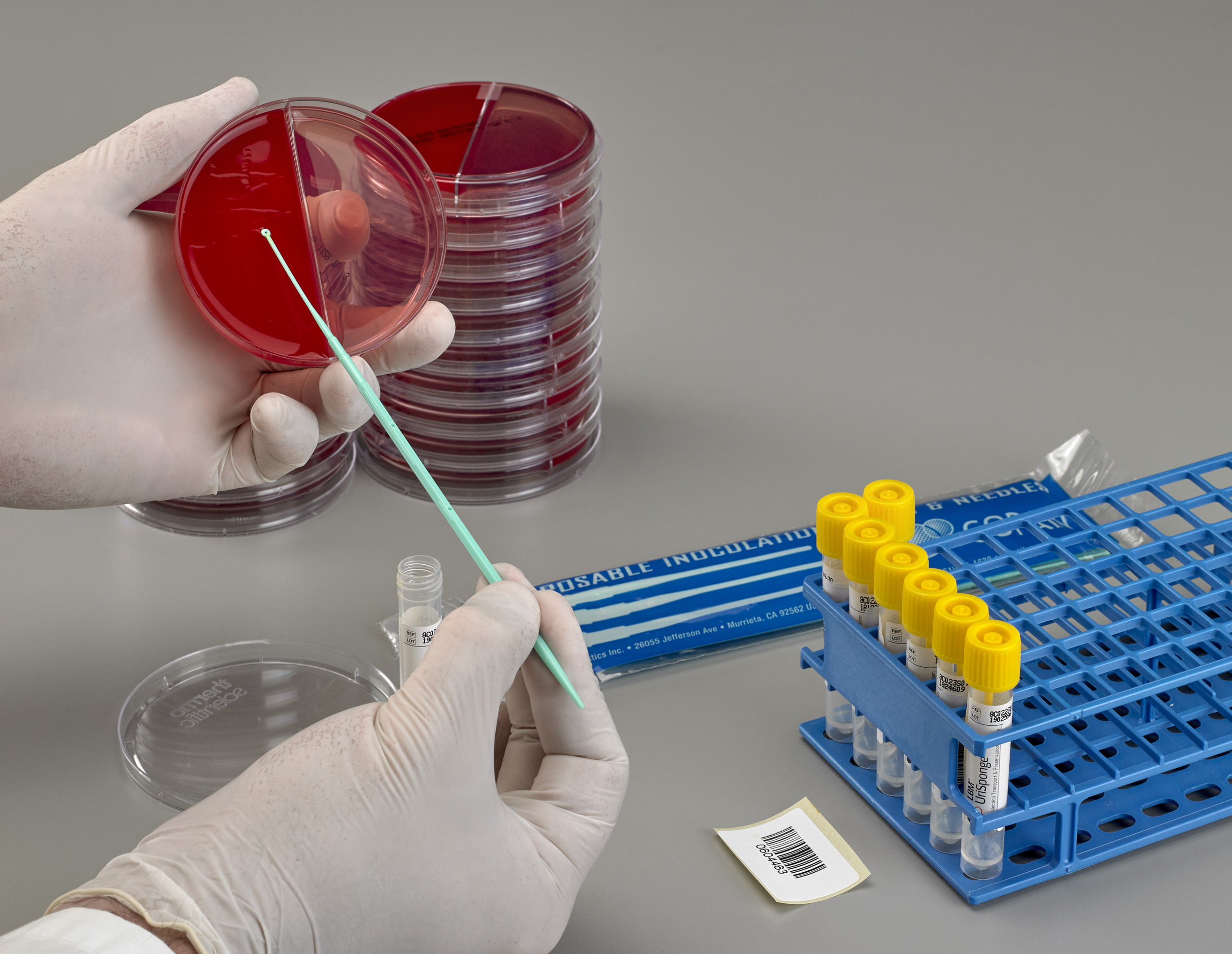
Is there training available for laboratories transitioning to UriSponge®?
Yes, Copan provides training resources to help laboratories implement UriSponge® seamlessly. Training materials, demonstrations, and ongoing support to ensure successful adoption and integration into your laboratory’s workflow.
Where can I get UriSponge®?
Copan received FDA clearance for the newly formulated UriSponge® in October 2024 and the system will be available through Copan’s U.S. distribution network in early 2025. Laboratories interested in adopting UriSponge™ can reach out to Copan for more information on ordering and implementation.


